Regulatory visits are an important tool of supervision, used by regulatory agencies and central banks in the UK and overseas. A visit typically lasts for a few days with regulators coming onsite, interviewing senior management and reviewing files. In the US, regulatory visits are often called ‘exams’.
Visits should be approached in exactly this way: as exams that can go either well or badly. Successful visits, where there is a polished performance, will give the regulators a positive impression and are likely to lead to lighter scrutiny or a less intensive regulatory programme. However, a bad visit can have all manner of negative consequences, including extra capital, S166 skilled persons reviews, expensive and burdensome additional scrutiny, and possible enforcement actions against the firm or individuals with senior responsibilities.
This blog highlights actions that will help you achieve a successful regulatory visit:
-
-
-
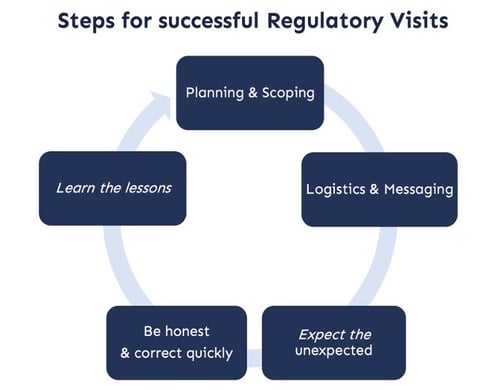
- Planning and preparation are half the battle
-
-
Benjamin Franklyn said, ‘By failing to prepare, you are preparing to fail’. You should approach each visit like a project and apply the usual project management disciplines. As with all projects, good planning is critical for a successful outcome – and it should start from the moment you receive the visit request letter. Understanding the visit scope and objectives is key, and this may need several calls with the supervisory team to gain clarity. It’s important to know who is on the supervisory visit team, what their specialisms are, and whether the team has a reputation for being particularly exacting.
-
-
-
- Finalise logistics and messaging
-
-
Ensuring that the key people are available on the agreed dates is critical. Just as importantly, everyone should be properly informed of the scope and context of the visit, and know what presentation materials have been prepared, reviewed, and approved by senior management. Compliance should ensure that interviewees are briefed on the day, including on issues arising from earlier meetings and on the style and approach of the supervision team.
Key takeaway messages should be developed and documented. For UK subsidiaries or branches of foreign firms, this may involve consultation with head office to ensure that answers provided to overseas regulators are accurate and consistent with answers provided to local regulators. Regulators are proficient at communicating with each other through supervisory colleges and bilateral meetings, so inconsistent messaging from firms is sure to raise concerns.
Don’t forget logistics such as rooms for the visit, equipment setups, and refreshments. It’s not a good idea to have the regulatory team in areas where they can hear staff chatting informally. Rooms should be organised for those waiting to be interviewed and for debriefing after their interview.
-
-
-
- Expect the unexpected
-
-
Don’t be surprised if the best-laid plans are scuppered on the day. This is usually because something ‘interesting’ is said in a meeting or the files open up a new field of investigation. Experienced supervisors may then decide to change the plan and speak to people who are not on the original agenda.
In addition, since the Senior Managers and Certification Regime (SM&CR) was introduced in the UK, regulators have deliberately interviewed members of staff who are one or even two levels below the designated SM&CR staff member, in order to better test the competence of certified persons. So be prepared for the supervisory team to interview more junior staff. Contrast this with the traditional visit where only the most senior staff (senior managers in today’s parlance) are interviewed. Our experienced former regulators at Chapelle can conduct mock interviews with senior managers and junior staff to provide an authentic experience of a regulatory visit. We will then provide feedback and advice through coaching.
-
-
-
- Be open and transparent – and correct errors quickly
-
-
Openness and transparency are essential when communicating with regulators. Supervisors can easily see through waffle or bluster and are experienced in triangulating between interviews to identify inconsistent responses. But regulators are human and will be sympathetic to reasonable explanations of why something has yet to be done, or if interviewees can’t provide statistics or other relevant information off the top of their heads. On the other hand, misleading the regulator is a very serious matter and will not be forgiven. If you realise after the meeting that you may have accidentally misled the regulator, it’s vital that you set the record straight. Your compliance team, or Chapelle’s experienced regulatory consultants, will be able to advise on the best course of action.
-
-
-
- Learn lessons for next time
-
-
An axiom of risk management is to learn from experience (especially mistakes) so that next time you can perform better. This is how it should be with regulatory visits. Once the visit is over, it’s important to have a have a thorough review and discuss honestly and openly what went well and what could have gone better.
With Nikhil Rathia as the new CEO at the Financial Conduct Authority and the regulator keen to respond to recent criticism of its approach to supervision, we expect the regulator to resume its onsite visit programme in earnest after lockdown, including for SM&CR and operational resilience.
If you found this blog useful, don't miss our 3 half-days Regulatory Visits Masterclass (on 29-30 September and 1 October 2021), taught by three former UK regulators, Lydia Bailey, Dr Jimi Hinchliffe and Andrew Sheen.
Further detail on this course is available here:
Chapelle consultants include experienced former regulators and regulatory compliance professionals who have conducted and managed numerous regulatory visits. Chapelle can assist you with training, mock visits (where we conduct interviews playing the role of regulators), and consulting support to help manage regulatory visits.
Reach out directly to find out more on how we can support your organisation:



